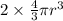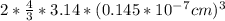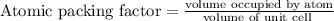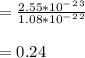Answers: 1


Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:02, jamiesong3501
What is the partial pressure of ethane, pethane, in the flask?
Answers: 1

Chemistry, 22.06.2019 10:00, emfranco1
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 12:30, AlexRavenwood127
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
Stannum has a body centered tetragonal with lattice constant, a = b = 5.83A and c = 3.18A. If the at...
Questions in other subjects: