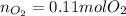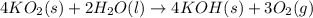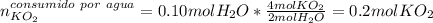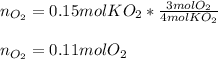oxígeno.

Chemistry, 28.05.2020 23:05 maceyshaynee9507
El superóxido de potasio, KO2, se emplea en máscaras de respiración para generar
oxígeno.
4KO2(s) + 2H2O(l) 4KOH(s) + 3O2(g)
Si un vaso de reacción contiene 0,15 mol de KO2 y 0,10 mol de H2O. ¿Cuál es el reactivo
limitante? ¿Cuántos moles de oxígeno se pueden producir?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, juliannxkim
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 17:30, ander67061
Air can be considered a mixture. which statement does not explain why?
Answers: 1


Chemistry, 23.06.2019 00:00, vanessacox45
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
You know the right answer?
El superóxido de potasio, KO2, se emplea en máscaras de respiración para generar
oxígeno.
oxígeno.
Questions in other subjects:

Mathematics, 19.05.2021 21:00

English, 19.05.2021 21:00



Health, 19.05.2021 21:00


Mathematics, 19.05.2021 21:00



Mathematics, 19.05.2021 21:00