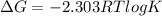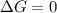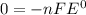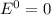Chemistry, 28.05.2020 22:04 wolfgirl2431
Given the chemical reaction: Hg2+(aq) + Cu(s) + Hg(s) + Cu2+(aq)
When the reaction reaches equilibrium, the cell potential will be
1
-0.51 V
2.
0.00 V
3.
0.51 V
4.
1.19 V

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:40, janelisse199820
Non renewable resources like petroleum eventually
Answers: 2
You know the right answer?
Given the chemical reaction: Hg2+(aq) + Cu(s) + Hg(s) + Cu2+(aq)
When the reaction reaches equ...
When the reaction reaches equ...
Questions in other subjects:



Mathematics, 20.11.2020 03:50

Mathematics, 20.11.2020 03:50

Mathematics, 20.11.2020 03:50