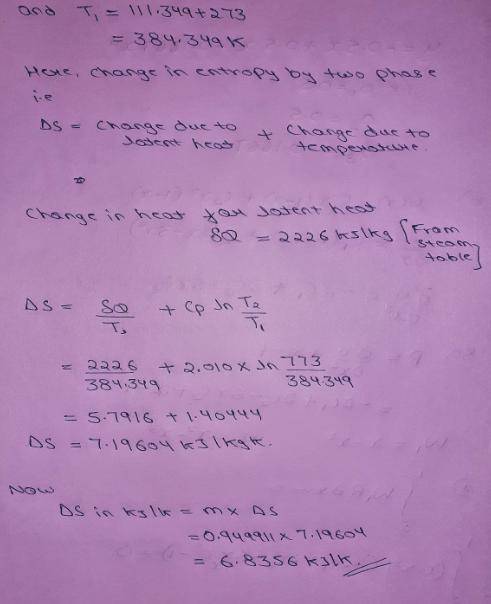Chemistry, 28.05.2020 19:01 donaldplawlerp5cctt
An insulated piston-cylinder device contains 1 L of saturated liquid water at pressure of 150 kPa. An electric resistance heater inside the cylinder is now turned on, and 2869.47 kJ of heat is transferred to the water. The inside H2O pressure maintains constant at 150 kPa during the process. Determine: the entropy change of the water during this heating process, in kJ/K.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, mamabates181981
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 06:10, Kianna000
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
You know the right answer?
An insulated piston-cylinder device contains 1 L of saturated liquid water at pressure of 150 kPa. A...
Questions in other subjects:

Mathematics, 04.08.2019 11:30


Mathematics, 04.08.2019 11:30


Spanish, 04.08.2019 11:30





Spanish, 04.08.2019 11:30