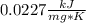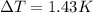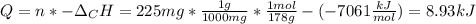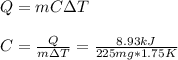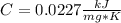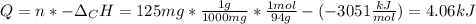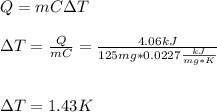Chemistry, 28.05.2020 18:57 robert7248
When 225mg of anthracene, C14H10(s), was burned in a bomb calorimeter the temperature rose by 1.75K. Calculate the calorimeter constant. By how much will the temperature rise when 125mg of phenol, C6H5OH(s), is burned in the calorimeter under the same conditions? (ΔcH<(C14H10,s)=–7061 kJ mol−1.)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, Ezekielcassese
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1
You know the right answer?
When 225mg of anthracene, C14H10(s), was burned in a bomb calorimeter the temperature rose by 1.75K....
Questions in other subjects:

Biology, 18.07.2019 18:00

History, 18.07.2019 18:00

History, 18.07.2019 18:00







History, 18.07.2019 18:00