
Chemistry, 28.05.2020 17:58 sofiaarmy12
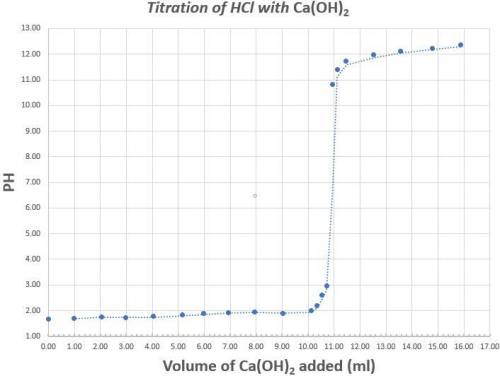
Read the graph to the nearest tenth for both pH and volume of calcium hydroxide. 25.0ml of a 1.70x10-4M solution of Hydrochloric acid was titrated with Calcium hydroxide. The above graph was generated when the Hydrochloric acid was titrated with Calcium hydroxide. Determine the concentration (in M) of the Calcium hydroxide. What is the coefficient of the scientific notation answer for the concentration of Calcium Hydroxide.
Determine the percent error if the known concentration of calcium hydroxide is 6.30x10-4M. (Do not put your answer in scientific notation).

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:10, ellemarshall13
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1


You know the right answer?
Read the graph to the nearest tenth for both pH and volume of calcium hydroxide. 25.0ml of a 1.70x10...
Questions in other subjects:







Chemistry, 05.05.2020 15:36

English, 05.05.2020 15:36

Mathematics, 05.05.2020 15:36



