
Chemistry, 28.05.2020 03:57 arianawelsh123l
"A gas turbine receives a mixture having the following molar analysis: 10% CO2, 19% H2O, 71% N2, at 720 K, 0.35 MPa and a volumetric flow rate of 3.2 m3 /s. The mixture exits the turbine at 380 K, 0.11 MPa. For adiabatic operation with negligible kinetic and potential energy effects, determine the power developed at steady state, in kW." NOTE: the process is NOT isentropic.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1


You know the right answer?
"A gas turbine receives a mixture having the following molar analysis: 10% CO2, 19% H2O, 71% N2, at...
Questions in other subjects:


History, 23.10.2019 19:30

History, 23.10.2019 19:30







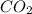 =0.1
=0.1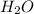 = O.19
= O.19
 =0.71
=0.71
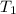 ) mixture receives from turbine =720K
) mixture receives from turbine =720K
 ) mixture receives from turbine =0.35 Mpa
) mixture receives from turbine =0.35 Mpa

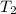 ) = 380K
) = 380K )= 0.11 Mpa
)= 0.11 Mpa
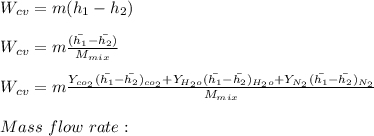
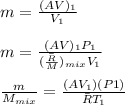
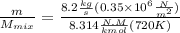



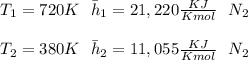
 2074.2 KW
2074.2 KW


