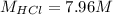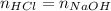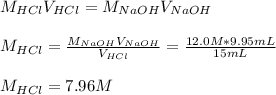Chemistry, 28.05.2020 01:06 psychocatgirl1
Concentrated hydrochloric acid is a solution that is 37.5% mass per unit volume HCl(aq) in water. An old bottle of HCl has an unknown concentration. What is the concentration of hydrochloric acid, [HCl], in the old bottle, if 9.95 mL of 12.0 M NaOH(aq) is required to reach the equivalence point when added to 15 mL of acid?What is the concentration of HCl(aq)?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, VictoriaRose520
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1


Chemistry, 22.06.2019 08:30, Blaise2653
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 18:30, chinadoll24
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
Concentrated hydrochloric acid is a solution that is 37.5% mass per unit volume HCl(aq) in water. An...
Questions in other subjects:

Mathematics, 24.07.2019 12:00



Biology, 24.07.2019 12:00


Physics, 24.07.2019 12:00

Mathematics, 24.07.2019 12:00



English, 24.07.2019 12:00