5. A standardized mass was known to weigh exactly 5.0000g. It was weighed on a newly
acquired,...

Chemistry, 28.05.2020 01:01 floreschachi2873
5. A standardized mass was known to weigh exactly 5.0000g. It was weighed on a newly
acquired, uncalibrated balance as 5.001g. Calculate the absolute error and the percent error.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2


Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3
You know the right answer?
Questions in other subjects:







Mathematics, 02.09.2019 17:50


English, 02.09.2019 17:50

Mathematics, 02.09.2019 17:50

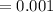
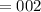 5
5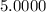 grams
grams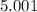 grams
grams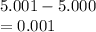
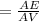
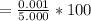
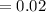 %
%

