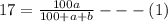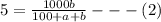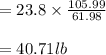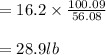Soda and lime are added to a glass batch in the form of soda ash (Na2CO3 ) and lime-stone (CaCO3 ). During heating, these two ingredients decompose to give off carbon dioxide (CO2 ), the resulting products being soda and lime. Compute the weight of soda ash and limestone that must be added to 125 lbm of quartz (SiO2 ) to yield a glass of composition 78 wt% SiO2 , 17 wt% Na2O, and 5 wt% CaO.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, noathequeen
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 12:30, poopybutt541
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 13:00, nauticatyson9
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
Soda and lime are added to a glass batch in the form of soda ash (Na2CO3 ) and lime-stone (CaCO3 )....
Questions in other subjects:



English, 10.11.2020 17:40




Mathematics, 10.11.2020 17:40