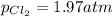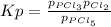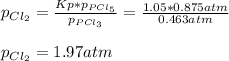Chemistry, 27.05.2020 23:06 hillarytrinh
3. The equilibrium constant KP for the decomposition of phosphorus pentachloride to phosphorus trichloride and molecular chlorine according to the chemical equation given below is found to be 1.05 at 250 °C. If the equilibrium partial pressures of PCl5 and PCl3 are 0.875 atm and 0.463 atm, respectively, what is the equilibrium partial pressure of Cl2 at 250 °C? PCl5(g) PCl3(g) + Cl2(g)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, KnMcdonaldk93906
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1



Chemistry, 22.06.2019 08:00, danielhall
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1
You know the right answer?
3. The equilibrium constant KP for the decomposition of phosphorus pentachloride to phosphorus trich...
Questions in other subjects:

Chemistry, 27.02.2021 19:00





Mathematics, 27.02.2021 19:00