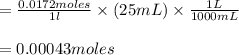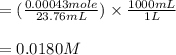Chemistry, 27.05.2020 21:07 gabriella80
2. 25.0mL aliquots of the solution in problem 1 are titrated with EDTA to the calmagite end point. A blank containing a small measured amount of Mg2 requires 2.12mL of the EDTA to reach the end point. An aliquot to which the same amount of Mg2 is added requires 25.88mL of the EDTA to reach the end point. a. How many mL of EDTA are needed to titrate the Ca2 ion in the aliquot

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:30, crystalbyrd79p8imrx
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

You know the right answer?
2. 25.0mL aliquots of the solution in problem 1 are titrated with EDTA to the calmagite end point. A...
Questions in other subjects:





Mathematics, 28.11.2019 10:31

Mathematics, 28.11.2019 10:31


English, 28.11.2019 10:31

Mathematics, 28.11.2019 10:31

Physics, 28.11.2019 10:31