
Chemistry, 27.05.2020 04:58 phsycotic121
What is the concentration of the silver ion in silver chromate, Ag₂CrO₄, if its solubility product constant (Kₛₚ) is 1.2 x 10⁻¹². Hint: write the equation first! *
2 points
1.4 x 10⁻⁵
1.1 x 10⁻⁹
1.6 x 10⁻¹²
2.4 x 10⁻¹²

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:20, Richwave17
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 20:00, 20calzoy
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 22.06.2019 21:30, sierradanielle9280
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 22.06.2019 21:30, rileydavidharless
Which substance can be broken down by chemical means
Answers: 1
You know the right answer?
What is the concentration of the silver ion in silver chromate, Ag₂CrO₄, if its solubility product c...
Questions in other subjects:

History, 07.01.2020 04:31



History, 07.01.2020 04:31


Social Studies, 07.01.2020 04:31

Chemistry, 07.01.2020 04:31

English, 07.01.2020 04:31


![[Ag^+]=1.3x10^{-4}M](/tpl/images/0666/7571/0a833.png)
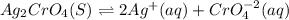
![Ksp=[Ag]^2[CrO_4^{-2}]](/tpl/images/0666/7571/75fed.png)
 due to the dissolution of silver chromate, we obtain:
due to the dissolution of silver chromate, we obtain:
![x=\sqrt[3]{\frac{1.2x10^{-12}}{2^2} } = 6.7x10^{-5}M](/tpl/images/0666/7571/1e8f3.png)
![[Ag^+]=2x=2*6.7x10^{-5}M=1.3x10^{-4}M](/tpl/images/0666/7571/1be44.png)


