
HW) Aspirin Poisoning
When your body fluids contain too much acid, it’s known as acidosis. Acidosis occurs when your kidneys and lungs can’t keep your body’s pH in balance. Many of the body’s processes produce acid. Your lungs and kidneys can usually compensate for slight pH imbalances, but problems with these organs can lead to excess acid accumulating in your body.
The acidity of your blood is measured by determining its pH. The pH of your blood should be around 7.4. According to the American Association for Clinical Chemistry (AACC), acidosis is characterized by a pH of 7.35 or lower. Alkalosis (too much base) characterized by a pH level of 7.45 or higher. While seemingly slight, these numerical differences can be serious.
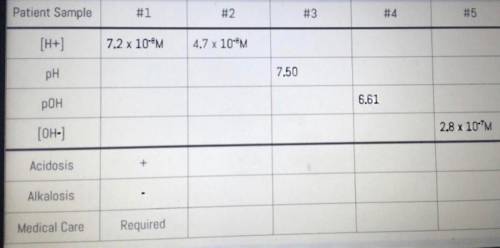
YOUR TASK: To determine whether or not the patient requires immediate medical care based on blood sample data.
The chart below represents blood sample data received from patients reporting aspirin overdose. Complete each patient’s chart and determine whether or not immediate medical care is necessary for metabolic acidosis and/or respiratory alkalosis. According to the AACC, normal blood pH ranges between 7.35 and 7.45. As evidence, you must show all your work.
Pls give me the answers
I’ll give brainliest answer
Please get them correct
Dude why is no one answering my question can someone answer it?!

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:20, jordan2875
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 23.06.2019 01:00, dawnparker71
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3

Chemistry, 23.06.2019 02:30, ijustneedhelp29
Which of the following statements are incorrect?
Answers: 3

Chemistry, 23.06.2019 06:30, aurikmah2005
Acertain atom has 22 protons and 19 electrons. this atom loses an electron. the net charge on the atom is now 4+1+01-4-. if this same atom with 22 protons and 19 electrons were to gain 3 electrons, the net charge on the atom would be 3+2+02-3-.
Answers: 1
You know the right answer?
HW) Aspirin Poisoning
When your body fluids contain too much acid, it’s known as acidosi...
When your body fluids contain too much acid, it’s known as acidosi...
Questions in other subjects:


English, 11.07.2019 16:30




English, 11.07.2019 16:30


Mathematics, 11.07.2019 16:30

History, 11.07.2019 16:30

Mathematics, 11.07.2019 16:30



