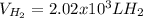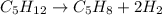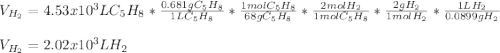Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, mathman783
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1


Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 18:00, rodriguezscarlet1713
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
How many liters of H2 can form when 4.53 x 10^3 mL C5H8 form?...
Questions in other subjects:






English, 18.03.2020 17:32



Mathematics, 18.03.2020 17:32