Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 20:00, bbyitskeke7160
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
You know the right answer?
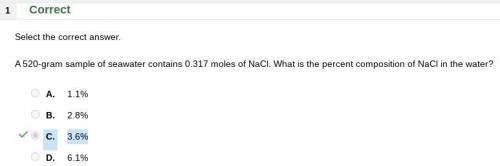
A 520-gram sample of seawater contains 0.317 moles of NaCl. What is the percent composition of NaCl...
Questions in other subjects:

Mathematics, 28.12.2020 21:10

Biology, 28.12.2020 21:10

History, 28.12.2020 21:10

History, 28.12.2020 21:10


Biology, 28.12.2020 21:10

Mathematics, 28.12.2020 21:10

Mathematics, 28.12.2020 21:10

Physics, 28.12.2020 21:10