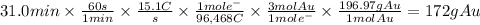Metal plating is done by passing a current through a metal solution. For example, an item can become gold plated by attaching the item to a power source and submerging it into an Au³⁺ solution. The item itself serves as the cathode, at which the Au³⁺ ions are reduced to Au(s). A piece of solid gold is used as the anode and is also connected to the power source, thus completing the circuit. What mass of gold is produced when 15.1 A of current are passed through a gold solution for 31.0 min?

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 12:00, kayla32213
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
You know the right answer?
Metal plating is done by passing a current through a metal solution. For example, an item can become...
Questions in other subjects:


English, 02.09.2019 02:30

Mathematics, 02.09.2019 02:30


Mathematics, 02.09.2019 02:30


Mathematics, 02.09.2019 02:30



Mathematics, 02.09.2019 02:30