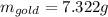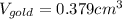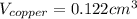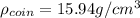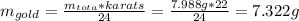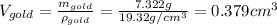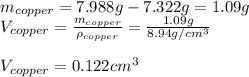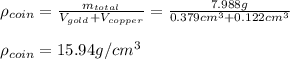Chemistry, 23.05.2020 18:58 fluffylove83
The British gold sovereign coin is an alloy of gold and copper having a total mass of 7.988 g, and is 22-karat gold.
(a) Find the mass of gold in the sovereign in kilograms using the fact that the number of karats = 24× (mass of gold)/ total mass.
(b) Calculate the volumes of gold and copper, respectively, used to manufacture the coin.
(c) Calculate the density of the British sovereign coin.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, mpchop
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 02:30, BornAdopted21
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 23.06.2019 06:40, Science2019
How many joules of heat are required to raise thetemperature of 750 g of water from 11.0 °c to 19.0 °c?
Answers: 1
You know the right answer?
The British gold sovereign coin is an alloy of gold and copper having a total mass of 7.988 g, and i...
Questions in other subjects:


Mathematics, 29.08.2019 22:30


Mathematics, 29.08.2019 22:30




Computers and Technology, 29.08.2019 22:40

Biology, 29.08.2019 22:40