Chemistry, 22.05.2020 06:58 josephvcarter
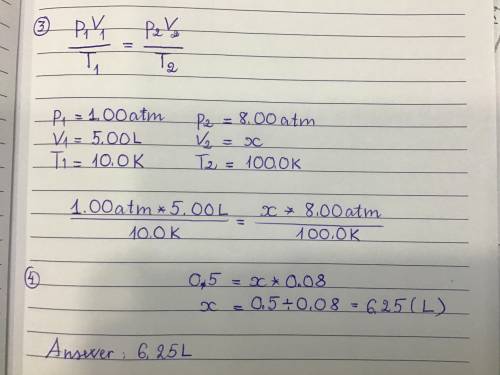
An ideal gas has a volume of 5.00L under a pressure of 1.00 atm and a temperature of 10.0 K. If the temperature is changed to 100.0 K while the pressure is increased to 8.00 atm what would be the new volume of the gas?
6.25L
1.60L
400.0L
16.0L

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, SchoolFirst9811
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н, о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2

Chemistry, 22.06.2019 12:00, WinterStrikesBack
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

You know the right answer?
An ideal gas has a volume of 5.00L under a pressure of 1.00 atm and a temperature of 10.0 K. If the...
Questions in other subjects:

Mathematics, 29.12.2019 07:31

History, 29.12.2019 07:31

Social Studies, 29.12.2019 07:31

History, 29.12.2019 07:31


Mathematics, 29.12.2019 07:31

Mathematics, 29.12.2019 07:31

History, 29.12.2019 07:31