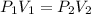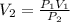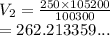Chemistry, 21.05.2020 05:09 tylerchitwood211
A 250.0mL sample of chlorine gas is collected when the barometric pressure is 105.2 kPa. What is the volume of the sample after the barometer drops to 100.3 kPa?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, thatonestudent2271
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 18:00, darrell1168
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 22.06.2019 21:30, thompsonhomes1
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 23.06.2019 07:00, kotetravels10
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1
You know the right answer?
A 250.0mL sample of chlorine gas is collected when the barometric pressure is 105.2 kPa. What is the...
Questions in other subjects:


Mathematics, 01.06.2021 09:40



Spanish, 01.06.2021 09:40

Physics, 01.06.2021 09:40

Computers and Technology, 01.06.2021 09:40


Geography, 01.06.2021 09:40