
Chemistry, 22.05.2020 01:00 ayoismeisjjjjuan
At high temperatures, calcium carbonate (CaCO3) decomposes to form calcium oxide (CaO) and carbon dioxide (CO2) gas. The average mass of 1 calcium oxide molecule is 56.079 amu, and the average mass of 1 carbon dioxide molecule is 44.009 amu.
CaCO3 CaO + CO2
Which is the total mass of the 1 calcium carbonate molecule?
A. 12.070 amu
B. 44.009 amu
C. 56.079 amu
D. 100.088 amu

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, coreyslotte
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2


Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2
You know the right answer?
At high temperatures, calcium carbonate (CaCO3) decomposes to form calcium oxide (CaO) and carbon di...
Questions in other subjects:

Chemistry, 12.11.2019 23:31

Physics, 12.11.2019 23:31







Geography, 12.11.2019 23:31

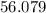 amu
amu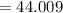 amu
amu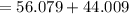
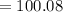 amu
amu

