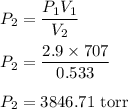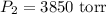Chemistry, 21.05.2020 23:58 washingtonisaia
A sample of chlorine gas occupies a volume of 707 ml at 2.90 atm. What is the new pressure in torr if the volume is compressed to 0.533 L?
a: 3,850 torr
b: 3.85 torr
c: 291 torr
d: 2,920 torr

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, connienash95
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 19:00, miguel454545
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 19:30, liyahlanderson2232
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2
You know the right answer?
A sample of chlorine gas occupies a volume of 707 ml at 2.90 atm. What is the new pressure in torr i...
Questions in other subjects:




Mathematics, 23.02.2021 21:50




History, 23.02.2021 21:50

Mathematics, 23.02.2021 21:50

Arts, 23.02.2021 21:50

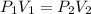
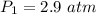
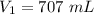
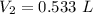
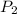 is the new pressure. So using Boyle's law we get :
is the new pressure. So using Boyle's law we get :