
Chemistry, 21.05.2020 22:08 rsimmons696
The ph of an aqueous solution at 25.0°c is 10.66. what is the molarity of h+ in this solution? the ph of an aqueous solution at 25.0°c is 10.66. what is the molarity of h+ in this solution? 4.6 à 10-4 3.3 2.2 à 10-11 1.1 à 10-13 4.6 à 1010

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, nuclearfire278
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease. correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 09:00, hellodarkness14
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1
You know the right answer?
The ph of an aqueous solution at 25.0°c is 10.66. what is the molarity of h+ in this solution? the...
Questions in other subjects:

Biology, 19.07.2019 13:00

Mathematics, 19.07.2019 13:00

English, 19.07.2019 13:00


Social Studies, 19.07.2019 13:00





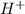 in this solution is
in this solution is 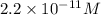
![pH=-\log [H^+]](/tpl/images/0659/9285/37e81.png)
![10.66=-\log[H^+]](/tpl/images/0659/9285/4a0df.png)
![[H^+]=10^{-10.66}](/tpl/images/0659/9285/0b8ff.png)
![[H^+]=2.2\times 10^{-11}](/tpl/images/0659/9285/b8988.png)


