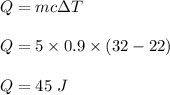Chemistry, 21.05.2020 17:57 StudentLife336
How many joules of heat are needed to raise the temperature of 5.0 g of aluminum from 22°C to 32°C, if the specific heat of aluminum is 0.90 J/g°C .

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 19:30, Karinaccccc
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 22.06.2019 20:30, dinapaul424
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 23.06.2019 03:10, mani1682
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2
You know the right answer?
How many joules of heat are needed to raise the temperature of 5.0 g of aluminum from 22°C to 32°C,...
Questions in other subjects:

English, 22.03.2021 05:20


Computers and Technology, 22.03.2021 05:20



History, 22.03.2021 05:20


Mathematics, 22.03.2021 05:20

English, 22.03.2021 05:20