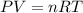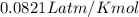Chemistry, 21.05.2020 07:59 kprincess16r
Find the heat produced from an 8.00 L cylinder of propane gas under 5.00 atm at 25.0 oC, if one mole of propane can produce 2220 kJ.
A. 4290 kJ
B. 0.0289 kJ
C. 877 kJ
D. 1.63 kJ
E. 5420 kJ
F. 1750 kJ
G. 8440 kJ
H. 1360 kJ
I. 37.2 kJ
J. 630 kJ
K. 266 kJ
L. 645 kJ
M. 2420 kJ
N. 7.36 x 10-4 kJ

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, MathChic68
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 23.06.2019 03:00, BeeShyanne
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1


You know the right answer?
Find the heat produced from an 8.00 L cylinder of propane gas under 5.00 atm at 25.0 oC, if one mole...
Questions in other subjects:



Advanced Placement (AP), 04.09.2020 14:01


Social Studies, 04.09.2020 14:01


Biology, 04.09.2020 14:01


Health, 04.09.2020 14:01

Health, 04.09.2020 14:01