Chemistry, 21.05.2020 06:59 elijahcarson9015
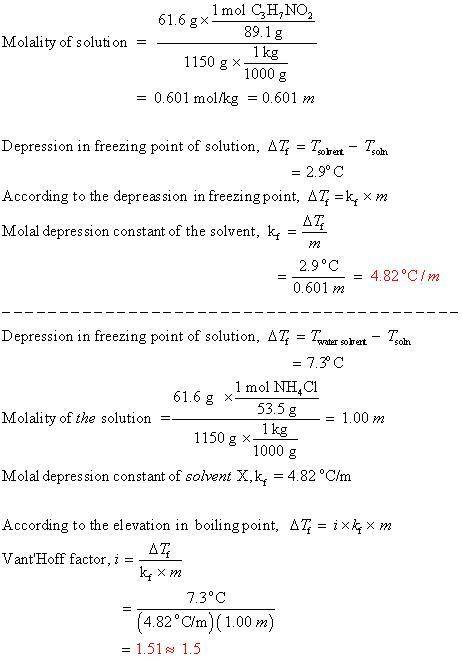
Question 1 When of alanine are dissolved in of a certain mystery liquid , the freezing point of the solution is lower than the freezing point of pure . On the other hand, when of iron(III) chloride are dissolved in the same mass of , the freezing point of the solution is lower than the freezing point of pure . Calculate the van't Hoff factor for iron(III) chloride in . Be sure your answer has a unit symbol, if necessary, and round your answer to significant digits.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, bartfrank447
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1


Chemistry, 22.06.2019 23:00, SophieCasey
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 23.06.2019 01:30, Sonicawesomeness
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
You know the right answer?
Question 1 When of alanine are dissolved in of a certain mystery liquid , the freezing point of the...
Questions in other subjects:


Mathematics, 21.02.2020 00:05

Mathematics, 21.02.2020 00:05




Mathematics, 21.02.2020 00:05