
Chemistry, 19.05.2020 22:17 12martinkat
Nswer the following questions relating to HCl, CH3Cl, and CH3Br. HCl(g), can be prepared by the reaction of concentrated H2SO4(ag), with NaCl(s), asa. represented by the following equation. H2SO4(ag) + 2 NaCl(s) → 2 HCl(g) Na2SO4(ag)A student claims that the reaction is a redox reaction. Is the student correct?i. Justify your answer. Calculate the mass, in grams, of NaCl(s), needed to react with excess H2SO4(ag)ii. to produce 3.0 grams of HCl(g). Assume that the reaction goes to completion

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, lilyclairehutson
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1


You know the right answer?
Nswer the following questions relating to HCl, CH3Cl, and CH3Br. HCl(g), can be prepared by the reac...
Questions in other subjects:


Mathematics, 13.11.2021 14:00




English, 13.11.2021 14:00

Mathematics, 13.11.2021 14:00




 remain in excess amount therefore NaCl (s) is the limiting reagent. Hence production of HCl entirely depends on amount of NaCl used.
remain in excess amount therefore NaCl (s) is the limiting reagent. Hence production of HCl entirely depends on amount of NaCl used.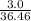 mol of HCl = 0.082 mol of HCl
mol of HCl = 0.082 mol of HCl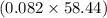 g = 4.8 g
g = 4.8 g

