
Chemistry, 19.05.2020 15:58 cameronrandom00
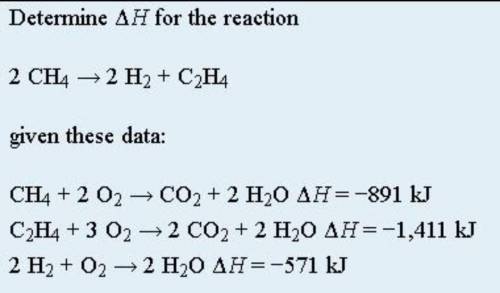
Hess’s law is very powerful. It allows us to combine equations to generate new chemical reactions whose enthalpy changes can be calculated, rather than directly measured. Besides that, Hess’s law states that when chemical equations are combined algebraically, their enthalpies can be combined in exactly the same way. Below points are to be taken as well: 1) If a chemical reaction is reversed, the sign on ΔH is changed; 2) If a multiple of a chemical reaction is taken, the same multiple of the ΔH is taken as well. As attached is an example of Hess Law problem solving question. By using Hess law , combine the equation algebraically and determine the enthaphy change of the reaction.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, drivinghydra
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1


You know the right answer?
Hess’s law is very powerful. It allows us to combine equations to generate new chemical reactions wh...
Questions in other subjects:



Mathematics, 12.11.2020 21:50

Mathematics, 12.11.2020 21:50

Social Studies, 12.11.2020 21:50



Mathematics, 12.11.2020 21:50


Computers and Technology, 12.11.2020 21:50



