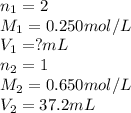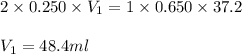Chemistry, 19.05.2020 03:15 evanwall91
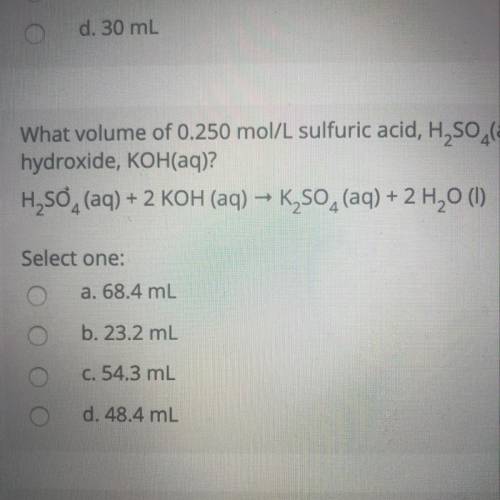
What volume of 0.250 mol/L sulfuric acid, H2SO4(aq) is needed to react completely with 37.2 mL of 0.650 mol/L potassium hydroxide, KOH(aq)?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, jalenshayewilliams
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1


Chemistry, 22.06.2019 02:00, webbhlharryteach
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 11:30, ashleybarrera2000
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3
You know the right answer?
What volume of 0.250 mol/L sulfuric acid, H2SO4(aq) is needed to react completely with 37.2 mL of 0....
Questions in other subjects:



Physics, 21.09.2019 17:30


Social Studies, 21.09.2019 17:30

Physics, 21.09.2019 17:30



Mathematics, 21.09.2019 17:30

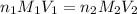
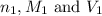 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 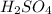
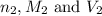 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.