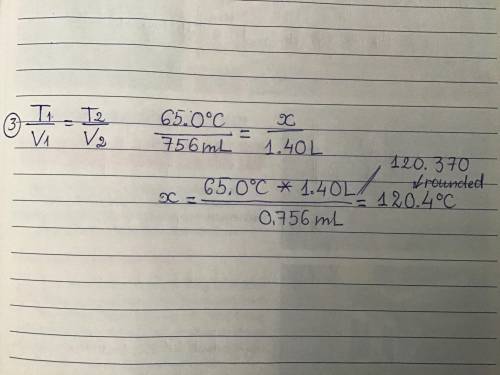Chemistry, 19.05.2020 03:04 matthewdabber7
At what temperature will a gas be, if you allow it to expand from its original volume of 756 mL at 65.0 °C to 1.40 L? (hint volume needs to be the same)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:10, bartonamber4042
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

You know the right answer?
At what temperature will a gas be, if you allow it to expand from its original volume of 756 mL at 6...
Questions in other subjects:

History, 19.02.2021 22:00

Mathematics, 19.02.2021 22:00

Mathematics, 19.02.2021 22:00


Mathematics, 19.02.2021 22:00


Mathematics, 19.02.2021 22:00

Mathematics, 19.02.2021 22:00