Calculate the [OH], [H30] and pOH given the following:
pH = 12.62
Show ALL work for...


Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, lucasrandall
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 04:40, khan2491
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 11:50, robert7248
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 23:00, maddyleighanne
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3
You know the right answer?
Questions in other subjects:

Chemistry, 04.12.2020 18:20


Arts, 04.12.2020 18:20



Mathematics, 04.12.2020 18:20

Mathematics, 04.12.2020 18:20

Mathematics, 04.12.2020 18:20


![[H_3O^+]=2.40x10^{-13}M](/tpl/images/0654/4727/6cc7e.png)
![[OH^-]=0.0417M](/tpl/images/0654/4727/c0526.png)
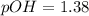
![[OH^-]=10^{-pOH}](/tpl/images/0654/4727/4817d.png)
![[H_3O^+]=10^{-pH}](/tpl/images/0654/4727/f57ec.png)
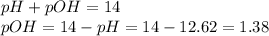
![[OH^-]=10^{-1.38}=0.0417M](/tpl/images/0654/4727/0dd3c.png)
![[H_3O^+]=10^{-12.62}=2.40x10^{-13}M](/tpl/images/0654/4727/e6e33.png)



