
Chemistry, 16.05.2020 10:57 shelbybibb99
An empty weighing dish has a mass of 1.0041 ± 0.0002 g. After you add dried sodium chloride to the dish, the mass is 3.2933 ± 0.0002 g. You quantitatively transfer the sodium chloride into a 500.00 ± 0.05 mL volumetric flask and dilute to the mark with deionized water. The molar mass of sodium chloride is 58.440 g/mol. What is the absolute error in the concentration of the resulting solution? Report your answer normally to the correct number of significant figures with the correct unit.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mayamabjishovrvq9
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 15:40, alleshia2007
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 16:00, corcoranrobert1959
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 23.06.2019 02:00, hayleebeals50
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2
You know the right answer?
An empty weighing dish has a mass of 1.0041 ± 0.0002 g. After you add dried sodium chloride to the d...
Questions in other subjects:

Biology, 11.09.2020 08:01

Mathematics, 11.09.2020 08:01

Mathematics, 11.09.2020 08:01

Mathematics, 11.09.2020 08:01

Mathematics, 11.09.2020 08:01

English, 11.09.2020 08:01

Mathematics, 11.09.2020 08:01

Mathematics, 11.09.2020 08:01

Mathematics, 11.09.2020 08:01

Mathematics, 11.09.2020 08:01

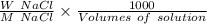
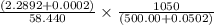
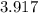 ±
± 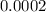 ) × (
) × (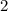 ±
± 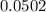 )
)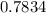 ±
± 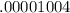 )
)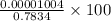
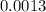 %
%

