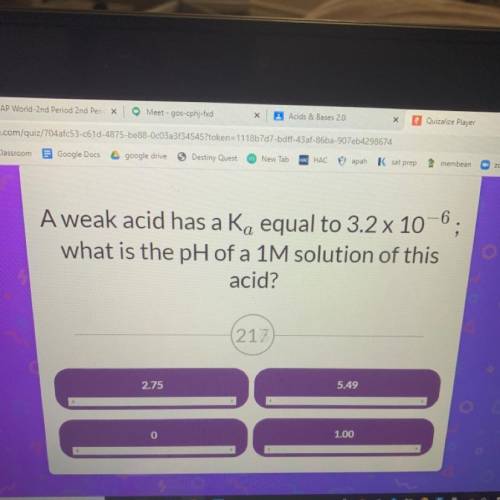
A weak acid has a Ka equal to 3.2 x 10^-6 what is the pH of a 1M solution of this acid?
...

Chemistry, 16.05.2020 11:57 khalidalrasheedi2025
A weak acid has a Ka equal to 3.2 x 10^-6 what is the pH of a 1M solution of this acid?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2


Chemistry, 22.06.2019 18:30, kate3887
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
Questions in other subjects:












