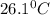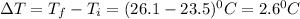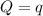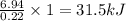Chemistry, 07.05.2020 05:16 rosepetals2938
The energy content of food is typically determined using a bomb calorimeter. Consider the combustion of a 0.22-g sample of butter in a bomb calorimeter having a heat capacity of 2.67 kJ/°C. If the temperature of the calorimeter increases from 23.5°C to 26.1°C, calculate the energy of combustion per gram of butter. Energy of combustion = kJ/g

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, tddreviews
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 14:30, isaiahrodriguezsm17
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 17:30, latezwardjr15
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
You know the right answer?
The energy content of food is typically determined using a bomb calorimeter. Consider the combustion...
Questions in other subjects:







Mathematics, 19.03.2020 19:40



Mathematics, 19.03.2020 19:40

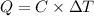
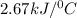
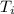 =
= 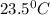
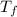 =
=