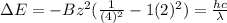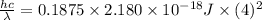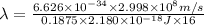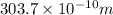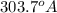Chemistry, 07.05.2020 05:15 granthazenp5e9mj
The energy of any one-electron species in its nth state (n = principal quantum number) is given by E = –BZ2 /n2 where Z is the charge on the nucleus and B is 18 2.180 10 J. a) Find the ionization energy of the Be3+ ion in its first excited state in kilojoules per mole. b) Find the wavelength of light given off from the Be3+ ion by electrons dropping from the fourth (n = 4) to the second (n = 2) energy levels.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 23:30, sweaversw9602
What are the similarities between compounds and mixtures?
Answers: 3
You know the right answer?
The energy of any one-electron species in its nth state (n = principal quantum number) is given by E...
Questions in other subjects:





English, 27.10.2020 18:00


History, 27.10.2020 18:00

English, 27.10.2020 18:00

Chemistry, 27.10.2020 18:00

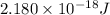
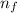 = 2; and
= 2; and 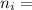 if it is ionized.
if it is ionized.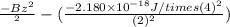
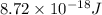
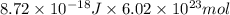
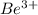 ion in its first excited state in kilojoules per mole is 5249 kJ/mol.
ion in its first excited state in kilojoules per mole is 5249 kJ/mol.