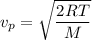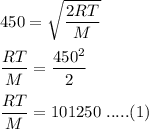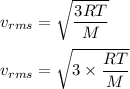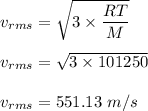Chemistry, 07.05.2020 04:58 justijust500
The most probable speed of an oxygen molecule in the gas phase at room temperature is 450 m/s. The root-mean-square speed (urms) is therefore equal to 450 m/s. much greater than 450 m/s. slightly less than 450 m/s. much less than 450 m/s. slightly greater than 450 m/s.

Answers: 3


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 10:50, lejeanjamespete1
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1
You know the right answer?
The most probable speed of an oxygen molecule in the gas phase at room temperature is 450 m/s. The r...
Questions in other subjects:






English, 23.07.2019 01:50