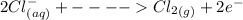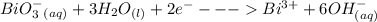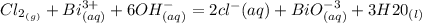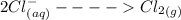Chemistry, 06.05.2020 20:11 owlette2001
Write balanced half-reactions for the following redox reactions
Cl2(g) + Bi3+ (aq) + 6OH-(aq) = 2cl-(aq) + BiO-3 (aq) + 3H20 (l)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, kkruvc
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 06:30, noathequeen
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 07:50, mckinleesmomp6qj1e
Which of the following electromagnetic waves can create ions?
Answers: 2
You know the right answer?
Write balanced half-reactions for the following redox reactions
Cl2(g) + Bi3+ (aq) + 6OH-(aq)...
Cl2(g) + Bi3+ (aq) + 6OH-(aq)...
Questions in other subjects:

Mathematics, 08.12.2020 02:00

English, 08.12.2020 02:00

Mathematics, 08.12.2020 02:00

Mathematics, 08.12.2020 02:00

Computers and Technology, 08.12.2020 02:00

Mathematics, 08.12.2020 02:00

Social Studies, 08.12.2020 02:00



Social Studies, 08.12.2020 02:00