Chemistry, 06.05.2020 20:11 vannybelly83
A volume of 60.0 mL of a 0.120 M HNO3 solution is titrated with 0.840 M KOH. Calculate the volume of KOH required to reach the equivalence point.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, huangjianhe135
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2



Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1
You know the right answer?
A volume of 60.0 mL of a 0.120 M HNO3 solution is titrated with 0.840 M KOH. Calculate the volume of...
Questions in other subjects:

Mathematics, 27.06.2019 00:00


Mathematics, 27.06.2019 00:00







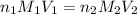
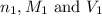 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 
 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.