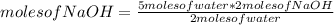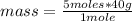How many grams of NaOH are produced with the reaction of 5.00 moles of water? *
2Na + 2H20 (ar...

Chemistry, 06.05.2020 19:09 emilystartk
How many grams of NaOH are produced with the reaction of 5.00 moles of water? *
2Na + 2H20 (arrow) 2NaOH +H2
50g
100g
200g
400g

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:10, sophiaa23
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 23.06.2019 01:30, kenldykido2300
Adirect relationship can be represented by: a curve a pie chart
Answers: 2
You know the right answer?
Questions in other subjects:

Mathematics, 08.03.2021 17:20

Geography, 08.03.2021 17:20



Mathematics, 08.03.2021 17:20



Mathematics, 08.03.2021 17:20


English, 08.03.2021 17:20