Given the equation representing a system at equilibrium in a sealed, rigid container:
2HI(g) &...

Given the equation representing a system at equilibrium in a sealed, rigid container:
2HI(g) <->H2(g) + I2(g) + energy
Increasing the temperature of the system causes the concentration of
1)HI to increase
2)H2 to increase
3) HI to Remain constant
4) HI to Remain constant

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, angelinararr5783
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1

Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 10:00, halohero7
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2
You know the right answer?
Questions in other subjects:


Chemistry, 01.09.2019 12:30

English, 01.09.2019 12:30




Chemistry, 01.09.2019 12:30

History, 01.09.2019 12:30


Biology, 01.09.2019 12:30


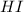 will increase.
will increase.

