
Match the following aqueous solutions with the appropriate letter from the column on the right. Assume complete dissociation of electrolytes. 1. 0.17 m NH4CH3COO A. Lowest freezing point 2. 0.18 m MnSO4 B. Second lowest freezing point 3. 0.20 m CoSO4 C. Third lowest freezing point 4. 0.42 m Ethylene glycol (nonelectrolyte) D. Highest freezing point

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 23:00, NewKidnewlessons
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 04:00, ayoismeisjjjjuan
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2
You know the right answer?
Match the following aqueous solutions with the appropriate letter from the column on the right. Assu...
Questions in other subjects:

Geography, 30.03.2021 15:10


Mathematics, 30.03.2021 15:10



Mathematics, 30.03.2021 15:10


Geography, 30.03.2021 15:10

Mathematics, 30.03.2021 15:10

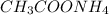 : Highest freezing point
: Highest freezing point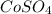 : Second lowest freezing point
: Second lowest freezing point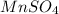 : Third lowest freezing point
: Third lowest freezing point 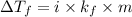
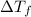 = change in freezing point
= change in freezing point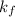 = freezing point constant
= freezing point constant


