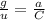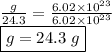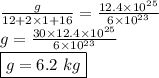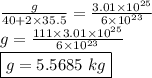Calculate the mass in grams of each of the following substances. 6.02 x 10²³ atoms of mg
...

Chemistry, 12.10.2019 20:30 deannajd03
Calculate the mass in grams of each of the following substances. 6.02 x 10²³ atoms of mg
calculate the mass in grams of each of the following substances.
12.4 x 10¹⁵ molecules of ch₂o
calculate the mass in grams of each of the following substances.
3.01 x 10²³ formula units of cacl₂

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:00, jaylanmahone223
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 22:30, angelagonzalesownus1
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
You know the right answer?
Questions in other subjects:


Mathematics, 22.04.2021 18:20






Business, 22.04.2021 18:20

History, 22.04.2021 18:20