
Chemistry, 05.05.2020 19:42 punkinrichard1oxon2i
A student does a titration with 14.80 mL of 0.225M H2SO4 and 25.00 mL of unknown LiOH solution. What is the molarity of the base Pls hurry

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 23:30, adamgala3885
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
You know the right answer?
A student does a titration with 14.80 mL of 0.225M H2SO4 and 25.00 mL of unknown LiOH solution. What...
Questions in other subjects:

Mathematics, 08.01.2021 23:40

Mathematics, 08.01.2021 23:40


Mathematics, 08.01.2021 23:40



Spanish, 08.01.2021 23:40

Spanish, 08.01.2021 23:40


History, 08.01.2021 23:40

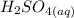 +
+ 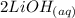 →
→ 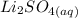 +
+ 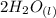
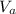 = 14.80 mL ,
= 14.80 mL , 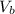 = 25.00 mL ,
= 25.00 mL , 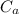 = 0.225M and
= 0.225M and 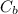 = ?
= ?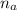 = 1 ,
= 1 , 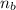 = 2
= 2

