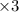Balance the redox reaction below using the half-reaction method. Sn2+(aq) + In(s)Sn(s) + In3+(aq) (a) To show your method, write the balanced half reactions below. Use the smallest integer coefficients possible and show electrons as e- . If a box is not needed, leave it blank. (Coefficients of 1 are not needed). Oxidation half-reaction: + + Reduction half-reaction: + + (b) To show your balanced equation, enter an integer in each of the boxes. If the integer is "1," do enter it even though you would normally not show that in the equation. Use the smallest integer coefficients possible. Sn2+(aq) + In(s) Sn(s) + In3+(aq)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, rileyeddins1010
List four observations that indicate that a chemical reaction may be taking place
Answers: 1



Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
Balance the redox reaction below using the half-reaction method. Sn2+(aq) + In(s)Sn(s) + In3+(aq) (a...
Questions in other subjects:





Mathematics, 22.06.2019 17:00



Spanish, 22.06.2019 17:00


Chemistry, 22.06.2019 17:00

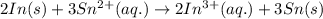
 .
. 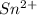 consume electrons and reduced to Sn.
consume electrons and reduced to Sn.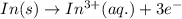 ]
]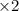
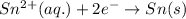 ]
]