
Chemistry, 05.05.2020 17:44 gapaxton22
The heat capacity of an object is given by the following equation: C equals 14000 straight J over straight K plus open parentheses 200 straight J over straight K squared close parentheses T plus open parentheses 3 straight J over straight K cubed close parentheses T squared What is the change in the entropy of the object (in J/K) associated with raising its temperature from 290 K to 380 K?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, angelteddy033
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 02:50, giiffnlojd
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

You know the right answer?
The heat capacity of an object is given by the following equation: C equals 14000 straight J over st...
Questions in other subjects:



Mathematics, 01.10.2019 20:00






Business, 01.10.2019 20:00

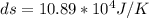
![C = 14000\frac{J}{K} + (200 \frac{J}{K^2} )T + [3 \frac{J}{K^3} ] T^2](/tpl/images/0640/9264/78425.png)
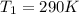
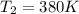

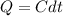
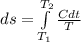
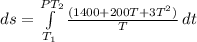
![ds = 1400\ ln [T]+ 200 \frac{T^2}{T} +3 \frac{T^2}{2 T} \ \ | \left \ T_2} \atop {T_1}} \right.](/tpl/images/0640/9264/c257a.png)
![ds = 1400 ln [\frac{T_2}{T_1} ] + 200 (T_2 - T_1 ) + \frac{3}{2} (T_2^2 -T_1^2)](/tpl/images/0640/9264/6b21c.png)
![ds = 1400 ln [\frac{380}{290} ] + 200 (380 - 290 ) + \frac{3}{2} (380^2 -290 ^2)](/tpl/images/0640/9264/2ea63.png)


