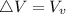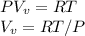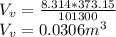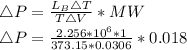Starting at atmospheric pressure, by how much must the pressure change in order to lower the boiling point of water by 1° C? You may assume that these changes are both small, so you only need to compute first derivatives. Remember that, under most conditions, the volume (per molecule) of liquid water is small compared to that of water vapor. Remember: Atmospheric pressure = 101300 Pa Boiling point at atmospheric pressure = 373.15 K Latent heat (LB) of boiling water = 2.256 × 106 J/kg Molecular weight (mw) of water = 0.018 kg/mol

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, JKINGblackstar3502
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 09:00, krystalhurst97
How are isotopes of the same chemical element alike? how are they different?
Answers: 1


Chemistry, 23.06.2019 04:31, mdarter
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2
You know the right answer?
Starting at atmospheric pressure, by how much must the pressure change in order to lower the boiling...
Questions in other subjects:

Mathematics, 27.01.2021 22:30

Mathematics, 27.01.2021 22:30

Biology, 27.01.2021 22:30


Biology, 27.01.2021 22:30

Mathematics, 27.01.2021 22:30




Mathematics, 27.01.2021 22:30

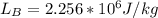
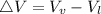
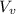 ) is far greater than the volume of liquid (
) is far greater than the volume of liquid (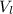 )
)