
Chemistry, 05.05.2020 16:44 ladnerhailey16
An equilibrium mixture of CO, O2 and CO2 at a certain temperature contains 0.0010 M CO2 and 0.0015 M O2. At this temperature, Kc equals 1.4 × 102 for the reaction: 2 CO(g) + O2(g) ⇌ 2 CO2(g). What is the equilibrium concentration of CO? An equilibrium mixture of CO, O2 and CO2 at a certain temperature contains 0.0010 M CO2 and 0.0015 M O2. At this temperature, Kc equals 1.4 × 102 for the reaction: 2 CO(g) + O2(g) ⇌ 2 CO2(g). What is the equilibrium concentration of CO? 3.1 × 10-1 M 4.8 × 10-6 M 2.2 × 10-3 M 1.9 × 10 7 M 9.3 × 10-2 M

Answers: 3


Other questions on the subject: Chemistry



You know the right answer?
An equilibrium mixture of CO, O2 and CO2 at a certain temperature contains 0.0010 M CO2 and 0.0015 M...
Questions in other subjects:

Geography, 24.10.2020 22:00


Mathematics, 24.10.2020 22:00



Mathematics, 24.10.2020 22:00

Physics, 24.10.2020 22:00

Mathematics, 24.10.2020 22:00

Mathematics, 24.10.2020 22:00

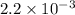 M.
M.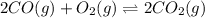
![K_{c}=\frac{[CO_{2}]^{2}}{[CO]^{2}[O_{2}]}](/tpl/images/0640/3657/11981.png)
![[CO_{2}]](/tpl/images/0640/3657/0a7e9.png) ,
, ![[CO]](/tpl/images/0640/3657/32558.png) and
and ![[O_{2}]](/tpl/images/0640/3657/9a638.png) represent equilibrium concentration of
represent equilibrium concentration of 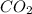 , CO and
, CO and 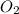 respectively.
respectively.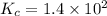
![[CO]=\sqrt{\frac{[CO_{2}]^{2}}{K_{c}.[O_{2}]}}](/tpl/images/0640/3657/02c20.png) M =
M = 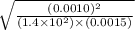 M =
M = 


