
Chemistry, 05.05.2020 09:46 kezionhoward13
Identify the correct set-up. How many moles of aluminum oxide would form if 25.0 g of aluminum react completely given the following chemical reaction: 4Al + 3O₂ → 2Al₂O₃?
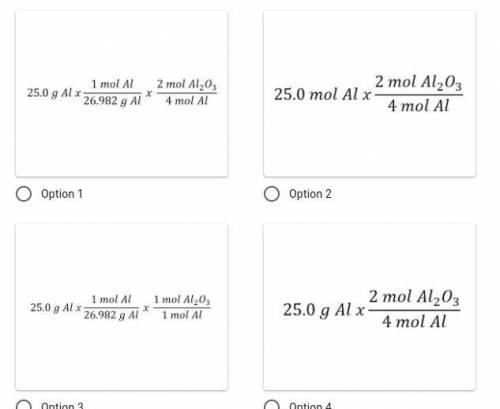

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, alydiale584
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 12:00, BreBreDoeCCx
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal. b. he is determining chemical properties that are sufficient to identify the metal. c. he is determining physical properties that are insufficient to identify the metal. d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 18:30, chinadoll24
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
Identify the correct set-up. How many moles of aluminum oxide would form if 25.0 g of aluminum react...
Questions in other subjects:


Social Studies, 30.07.2019 01:50


History, 30.07.2019 01:50

Geography, 30.07.2019 01:50



Mathematics, 30.07.2019 01:50

Chemistry, 30.07.2019 01:50



