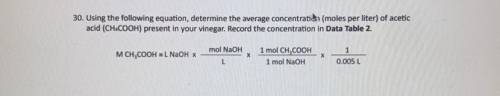Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, kev71
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 02:00, issachickadi
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 02:30, bionicboy03120440
What is the mass of sodium in 3 moles of sodium chloride
Answers: 1

Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3
You know the right answer?
Using The following equation, determine the average concentration (mole per liter) of acetic acid (C...
Questions in other subjects:

Mathematics, 19.08.2019 12:20






Mathematics, 19.08.2019 12:20

History, 19.08.2019 12:20


Mathematics, 19.08.2019 12:20