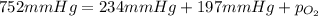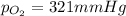A mixture of helium, nitrogen, and oxygen has a total pressure of 752 mm Hg. The
partial press...

Chemistry, 05.05.2020 05:42 tylorroundy
A mixture of helium, nitrogen, and oxygen has a total pressure of 752 mm Hg. The
partial pressures of helium and nitrogen are 234 mm Hg and 197 mm Hg, respectively.
What is the partial pressure of oxygen in the mixture?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, Jerrikasmith28
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 06:00, hdjsjfjruejchhehd
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

You know the right answer?
Questions in other subjects:


English, 10.12.2020 14:00


Mathematics, 10.12.2020 14:00


History, 10.12.2020 14:00

Mathematics, 10.12.2020 14:00

Mathematics, 10.12.2020 14:00


Mathematics, 10.12.2020 14:00

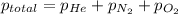
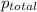 = total pressure of gases = 752 mm Hg
= total pressure of gases = 752 mm Hg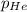 = partial pressure of Helium = 234 mm Hg
= partial pressure of Helium = 234 mm Hg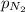 = partial pressure of nitrogen = 197 mm Hg
= partial pressure of nitrogen = 197 mm Hg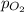 = partial pressure of oxygen = ?
= partial pressure of oxygen = ?